What is true of a mixture brainpop – Welcome to our exploration of the fascinating world of mixtures! What is true of a mixture? BrainPOP takes us on a captivating journey to uncover the secrets of these unique combinations of substances.
Mixtures, found all around us, are formed when two or more substances blend together, retaining their individual identities. From the air we breathe to the beverages we sip, mixtures play a crucial role in our daily lives.
Definition of a Mixture

A mixture is a combination of two or more chemical substances that are not chemically bonded. The substances retain their identity and are mixed in different forms, for example, solutions, suspensions, or colloids. Mixtures are created by mechanically blending or mixing elements and compounds without any chemical bonding or change so that each ingredient retains its composition and its own unique properties.
Types of Mixtures
Mixtures can be classified into two main types based on their composition and appearance:
- Homogeneous Mixtures:In a homogeneous mixture, the components are evenly distributed throughout the mixture, resulting in a uniform composition and appearance. Examples include saltwater, air, and alloys.
- Heterogeneous Mixtures:In a heterogeneous mixture, the components are not evenly distributed, resulting in a non-uniform composition and appearance. Examples include sand in water, oil in water, and granite.
Components of a Mixture
A mixture is a combination of two or more chemical substances that are not chemically bonded. The substances retain their identity and are mixed in different forms, for example, solutions, suspensions, or colloids.
Mixtures are composed of various components, each playing a specific role in determining the overall properties of the mixture.
Solvents and Solutes
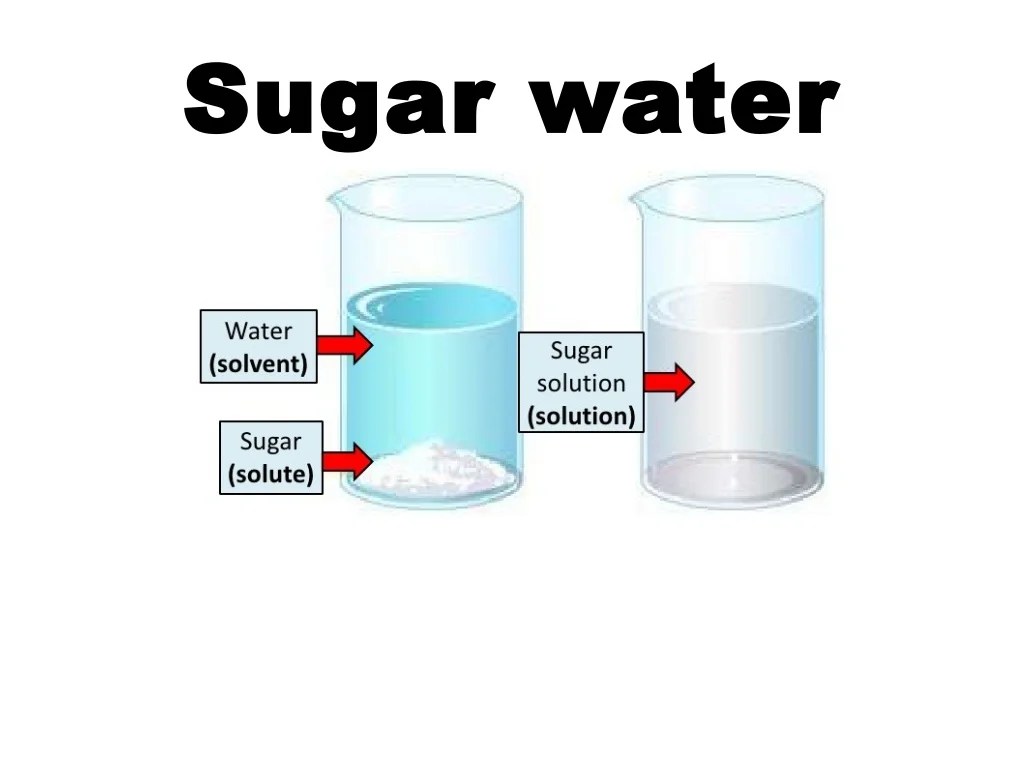
In a solution, the component present in the largest amount is called the solvent, while the component present in a smaller amount is called the solute. The solvent dissolves the solute, forming a homogeneous mixture.
For example, in a saltwater solution, water is the solvent, and salt is the solute. The water molecules surround and separate the salt molecules, allowing them to disperse evenly throughout the solution.
Proportions of Components
The proportions of the components in a mixture can significantly affect its properties. For instance, increasing the concentration of a solute in a solution can alter its freezing point, boiling point, and other physical characteristics.
In a suspension, the proportions of the components determine the stability and settling rate of the particles. A higher concentration of particles can lead to a faster settling rate, while a lower concentration can result in a more stable suspension.
Physical Properties of Mixtures: What Is True Of A Mixture Brainpop

Mixtures exhibit unique physical properties that differ from those of their pure components. Understanding these properties is crucial for comprehending mixture behavior and developing separation techniques.
The physical properties of mixtures are influenced by the properties of their components and the proportions in which they are combined. Factors such as molecular weight, polarity, and intermolecular forces play significant roles in determining mixture properties.
Boiling Point
- The boiling point of a mixture is typically lower than the boiling point of its most volatile component.
- As the proportion of the volatile component increases, the boiling point of the mixture decreases.
- This property can be utilized in fractional distillation, a technique used to separate liquids based on their boiling points.
Freezing Point, What is true of a mixture brainpop
- The freezing point of a mixture is typically lower than the freezing point of its pure components.
- The presence of impurities or other components can disrupt the crystal lattice formation, lowering the freezing point.
- This property is exploited in antifreeze solutions, which prevent water from freezing in car engines.
Density
- The density of a mixture is often a weighted average of the densities of its components.
- Mixtures with higher proportions of denser components will have higher overall densities.
- Density differences can be used in separation techniques such as centrifugation and floatation.
Chemical Properties of Mixtures
Mixtures inherit the chemical properties of their components. Reactivity and flammability are two important chemical properties to consider.
- Reactivityrefers to the ability of a substance to undergo chemical reactions. The reactivity of a mixture depends on the reactivity of its components. For example, a mixture of hydrogen and oxygen is highly reactive and can explode when ignited.
- Flammabilityrefers to the ability of a substance to catch fire and burn. The flammability of a mixture depends on the flammability of its components. For example, a mixture of gasoline and air is highly flammable and can easily catch fire.
Chemical reactions can occur in mixtures. For example, when hydrogen and oxygen are mixed, they can react to form water. This reaction is highly exothermic, meaning that it releases heat.
Separation Techniques for Mixtures
Separating mixtures into their components is crucial in various scientific and industrial applications. Numerous techniques have been developed to achieve this, each exploiting specific physical or chemical properties of the mixture’s components.
Filtration
Filtration is a simple yet effective method used to separate solid particles from liquids or gases. A filter paper or membrane with pores of a specific size is used to allow the liquid or gas to pass through while retaining the solid particles.
This technique is commonly employed in water purification, air filtration, and coffee brewing.
Distillation
Distillation is a process that separates liquids based on their different boiling points. The mixture is heated, and the components with lower boiling points vaporize first. These vapors are then condensed and collected, resulting in the separation of the liquids.
Distillation is widely used in the production of alcoholic beverages, essential oils, and petroleum refining.
Chromatography
Chromatography is a versatile technique that separates components of a mixture based on their different affinities for a stationary and a mobile phase. The mixture is introduced into the stationary phase, and the mobile phase is passed through it. Components with different affinities for the two phases will travel at different rates, allowing for their separation.
Chromatography is used in various fields, including analytical chemistry, biochemistry, and drug development.
General Inquiries
What is the difference between a homogeneous and heterogeneous mixture?
A homogeneous mixture is uniform throughout, with its components evenly distributed. In contrast, a heterogeneous mixture displays visible variations in composition.
How can we separate mixtures?
Various techniques exist for mixture separation, including filtration, distillation, and chromatography, each exploiting specific properties of the mixture’s components.
Why is understanding mixtures important?
Mixtures are ubiquitous in our world, and comprehending their behavior helps us navigate various scientific and practical applications, from medicine to environmental science.