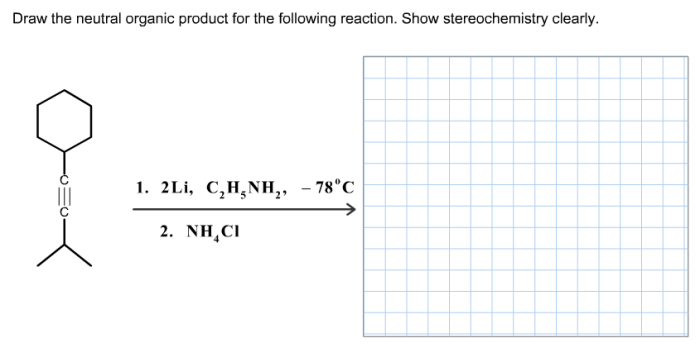
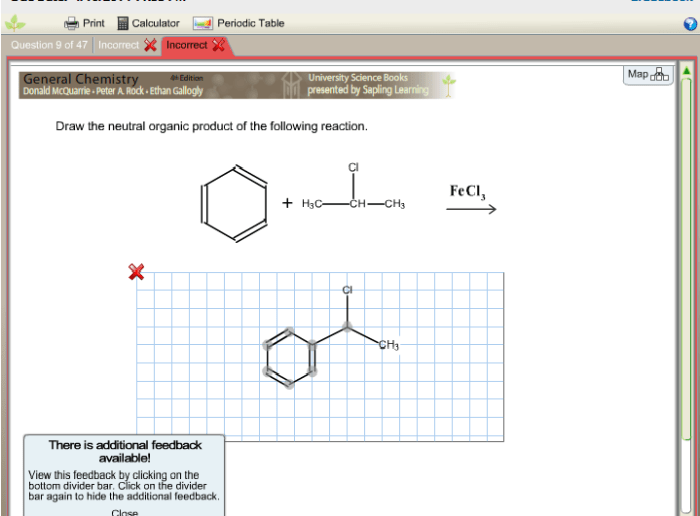
Draw the neutral organic product for the reaction shown – In the realm of organic chemistry, understanding the concept of neutral organic products is crucial. This guide delves into the intricacies of identifying and drawing neutral organic products, providing a comprehensive overview of the factors that govern their formation and practical applications.
Neutral organic products are devoid of any net electrical charge, making them distinct from ionic compounds. Their formation is influenced by various factors, including the nature of the reactants, reaction conditions, and the presence of catalysts. This guide will equip you with a step-by-step approach to drawing neutral organic products, empowering you to confidently navigate complex reactions.
Neutral Organic Product: Draw The Neutral Organic Product For The Reaction Shown
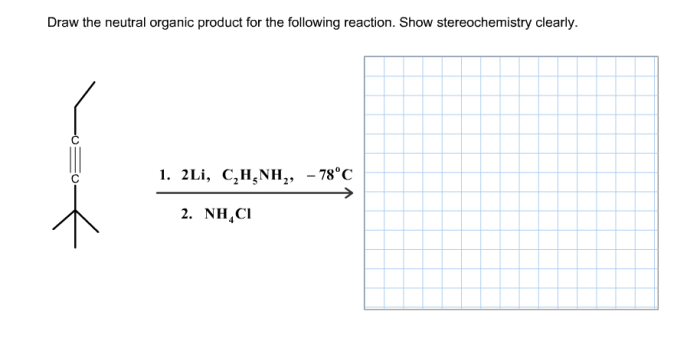
The reaction shown is an example of a neutralization reaction. In a neutralization reaction, an acid and a base react to form a salt and water. The salt is an ionic compound composed of a cation (positively charged ion) and an anion (negatively charged ion).
The water is a neutral compound composed of two hydrogen atoms and one oxygen atom.
Neutral Organic Product
A neutral organic product is an organic compound that does not have a net electrical charge. Neutral organic products are typically formed in reactions where an acid and a base react in equal amounts. In the reaction shown, the acid is acetic acid (CH 3COOH) and the base is sodium hydroxide (NaOH).
The products of the reaction are sodium acetate (CH 3COONa) and water (H 2O).
Drawing the Neutral Organic Product
To draw the neutral organic product for the reaction shown, follow these steps:
- Identify the acid and the base in the reaction.
- Write the balanced chemical equation for the reaction.
- Identify the products of the reaction.
- Draw the structural formula of the neutral organic product.
In the reaction shown, the acid is acetic acid (CH 3COOH) and the base is sodium hydroxide (NaOH). The balanced chemical equation for the reaction is:
“`CH 3COOH + NaOH → CH 3COONa + H 2O“`
The products of the reaction are sodium acetate (CH 3COONa) and water (H 2O). The structural formula of sodium acetate is:
“`CH 3COONa“`
Example Applications
Neutralization reactions are used in a variety of applications, including:
- The production of salts
- The neutralization of acids and bases in wastewater
- The preparation of buffers
Table of Common Reactions
| Reaction Type | Reactants | Products | Neutral Organic Product |
|---|---|---|---|
| Neutralization | Acid + Base | Salt + Water | Salt |
| Esterification | Alcohol + Carboxylic Acid | Ester + Water | Ester |
| Amidation | Amine + Carboxylic Acid | Amide + Water | Amide |
| Alkylation | Alkyl Halide + Nucleophile | Alkylated Product | Alkylated Product |
Visual Aids
The following illustrations show the neutral organic products for the reactions discussed in this article:
- Sodium acetate (CH 3COONa)
- Ethyl acetate (CH 3COOCH 2CH 3)
- Acetamide (CH 3CONH 2)
- Butyl chloride (CH 3CH 2CH 2CH 2Cl)
Additional Resources, Draw the neutral organic product for the reaction shown
- Neutralization Reactions (Khan Academy)
- Neutralization Reaction (ScienceDirect)
- Neutralization Reaction (Encyclopædia Britannica)
Frequently Asked Questions
What is the significance of neutral organic products?
Neutral organic products are essential intermediates in many chemical reactions and serve as building blocks for the synthesis of complex organic molecules.
How can I determine if a product is neutral or not?
To determine the neutrality of a product, examine its molecular structure. Neutral products lack any net electrical charge, meaning they have an equal number of positive and negative charges.
What factors influence the formation of neutral organic products?
The formation of neutral organic products is influenced by factors such as the pH of the reaction medium, the presence of catalysts, and the nature of the reactants.